How To Turn Iron Into Steel
After the pig iron has been tapped, it must be desulfurized and oxidized with oxygen (refining) to obtain crude steel.
- one Introduction
- two Desulfurization
- 3 Decarburization (basic oxygen steelmaking)
Introduction
Due to its high carbon content and relatively high concentrations of phosphorus and sulphur, pig iron is by and large very brittle and is non suitable for forging or welding. Therefore, the squealer iron must be postal service-treated to produce the actual steel with its typical forging and welding properties. This aftertreatment takes identify in steelworks (steel mill) whose main task is to reduce carbon to the desired level of less than 2 % and to remove the contaminants such as sulphur, phosphorus and nitrogen as far as possible (and, to a certain extent, silicon and manganese).
The sulphur has a specially detrimental effect. It leads to the germination of iron sulfide, which forms a low-melting mixture in combination with oxygen. The iron sulfide segregates at the grain boundaries of the austenite grains during subsequent hot forming in the range of 800 °C to 1000 °C and thus leads to embrittlement of the microstructure. Since the fracture surface of a steel broken at these temperatures is red-hot, this type of fracture is also known equally ruby shortness. From a temperature of 1200 °C the iron sulfide inclusions even begin to cook and thus too pb to breakage. This is then referred to as hot shortness.
In guild to avert red and hot shortness, steels must therefore more often than not be low in sulphur (exception: free machining steel). In addition, manganese is normally added, which then binds the sulphur to itself in the course of manganese sulphide and is therefore non present in the harmful class of iron sulphide.
In the following sections, the reduction of sulphur past targeted desulphurisation and the reduction of carbon content by refining through oxidation are therefore discussed in more detail.
Pig iron is likewise breakable to exist used as a construction textile due to its high carbon and sulphur content! Pig iron must therefore be desulphurised and decarburised!
Desulfurization
The refining of pig iron into steel is carried out in liquid form. For this purpose, the pig iron produced in the ironworks is commencement collected in large mixing containers (chosen hog iron mixers) with a capacity of up to 1800 tons. Several squealer iron tappings are mixed in it to compensate for differences in composition and thus provide the steelworks with a constant quality.
With manganese-rich hog fe, pre-desulfurization already takes place without any farther activeness, equally the sulfur has a greater affinity to manganese than to iron. Ferromanganese additives enhance this effect. During this so-called manganese desulfurization, the iron sulfide (\(FeS\)) forms manganese sulfide (\(MnS\)) in the pig fe melt:
\brainstorm{align}
\label{manganentschwefelung}
&FeS ~+~ Mn \rightleftharpoons~ Fe ~+~ MnS ~~~~~~ \text{(exothermic)} \\[5px]
\end{align}
The manganese sulphide, which is insoluble in liquid pig iron, is deposited equally slag on the melt. In principle, however, information technology is non possible to remove all the sulphur from the melt considering the reaction equilibrium is shifted to the left at high temperatures (which are necessary to keep the squealer iron liquid).
For this reason, calcium oxide or calcium carbide is added to the pig iron earlier it is transported to or in the steel mill and thus desulfurized (chosen lime desulfurization). The iron sulfide (\(FeS\)) independent in liquid hog iron essentially reacts with the calcium oxide (\(CaO\)) or calcium carbide (\(CaC_2\)) to form calcium sulfide (\(CaS\)). Magnesium oxide (\(MgO\)) has the same desulfurizing upshot. High temperatures in detail favour this form of desulphurisation. The chemical equations for this are equally follows:
\begin{marshal}
\characterization{kalkentschwefelung}
&FeS ~+~ CaO &&\rightleftharpoons~ FeO && +~ CaS \\[5px]
&FeS ~+~ CaC_2 &&\rightleftharpoons~ Fe &&+~ CaS ~+~ 2~C \\[5px]
&FeS ~+~ MgO &&\rightleftharpoons~ FeO &&+~ MgS \\[5px]
\end{align}
The calcium or magnesium sulphide formed is then jump in a basic slag. Basically, only slags fabricated of basic compounds can demark acidic substances such as sulphur (and phosphorus). Depending on the process, the sulfur content in squealer iron can exist reduced to up to 0.001 %.
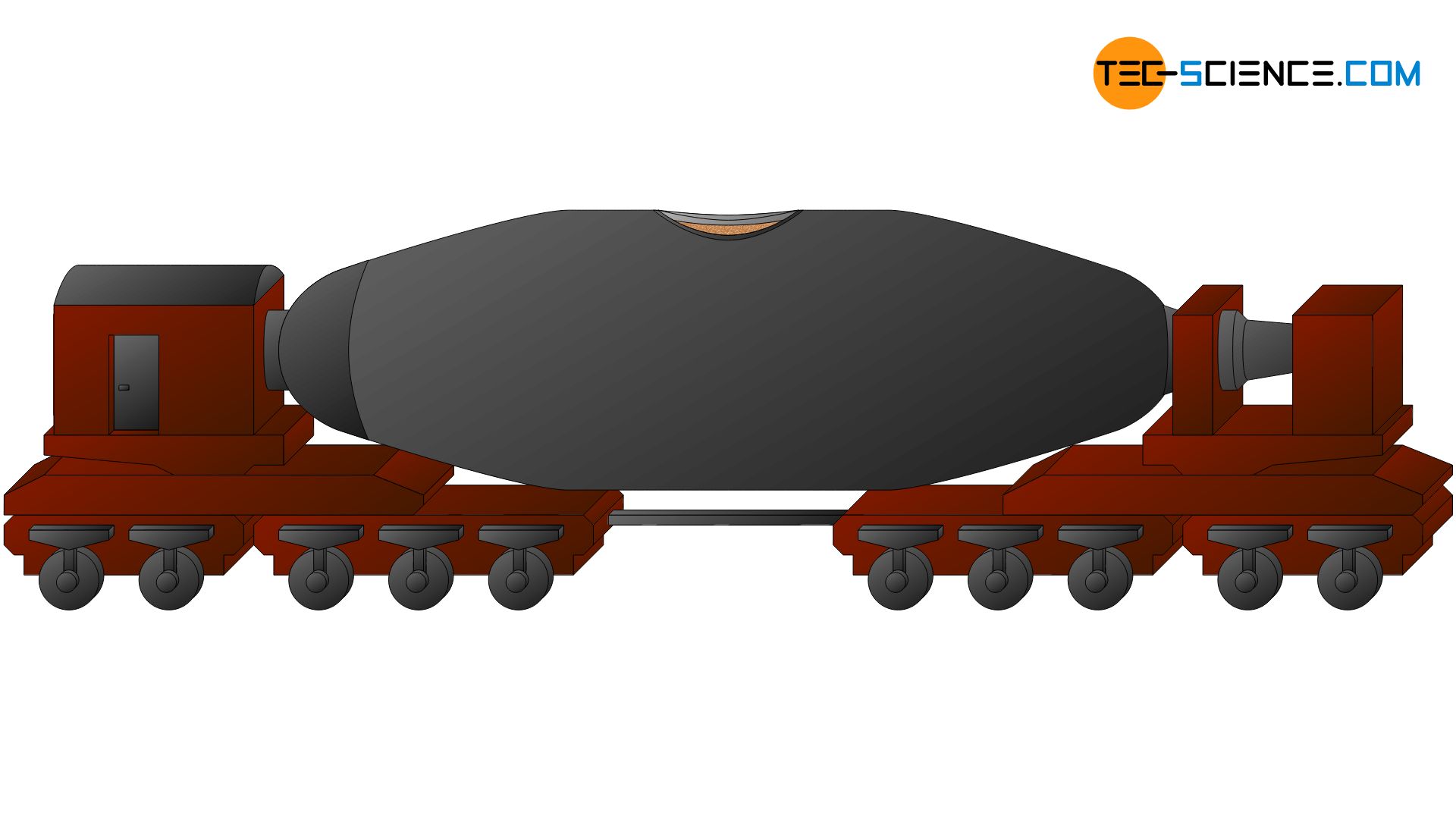
The largely desulphurised hog iron is further candy in the steel mill itself. If it is non an integrated steelworks, the pig fe may have to exist transported several kilometres from the smelter to the steel factory. This usually takes place in then-chosen torpedo cars, which are located on railway tracks.
This ways of ship owes its name to its elongated torpedo shape. The capacity of such a torpedo car is about 300 tons. The refractory lined wagons permit the squealer iron to cool merely minimally on its fashion to the steelworks (approx. 10 °C per hr). The still molten steel can then be further processed in the steelworks.
Decarburization (bones oxygen steelmaking)
Once the molten squealer iron has arrived in the steelworks later desulfurization, information technology is farther processed into (crude) steel. At starting time one makes use of that the accompanying elements, such equally phosphorus, which are yet present in undesirable amounts, just too elements such as carbon, silicon and manganese, which are nowadays in excessive concentrations, accept a greater affinity to oxygen than iron.
This offers the possibility of burning the accompanying elements in the liquid squealer iron relatively simply with the supply of oxygen. This causes the unwanted substances to oxidise and are bound or gasified in a slag-forming layer. In contrast to sus scrofa fe production in the blast furnace, the combustion process at this point does not involve carbon, as this is to be partially removed from the pig atomic number 26, among other things.
In the past, fresh air was blown into the pig atomic number 26 cook for oxidation. In the course of fourth dimension, different refined processes have developed, many of which are no longer upward to appointment. Therefore, the most of import process, the oxygen converter process (basic oxygen steelmaking), is described in more particular below.
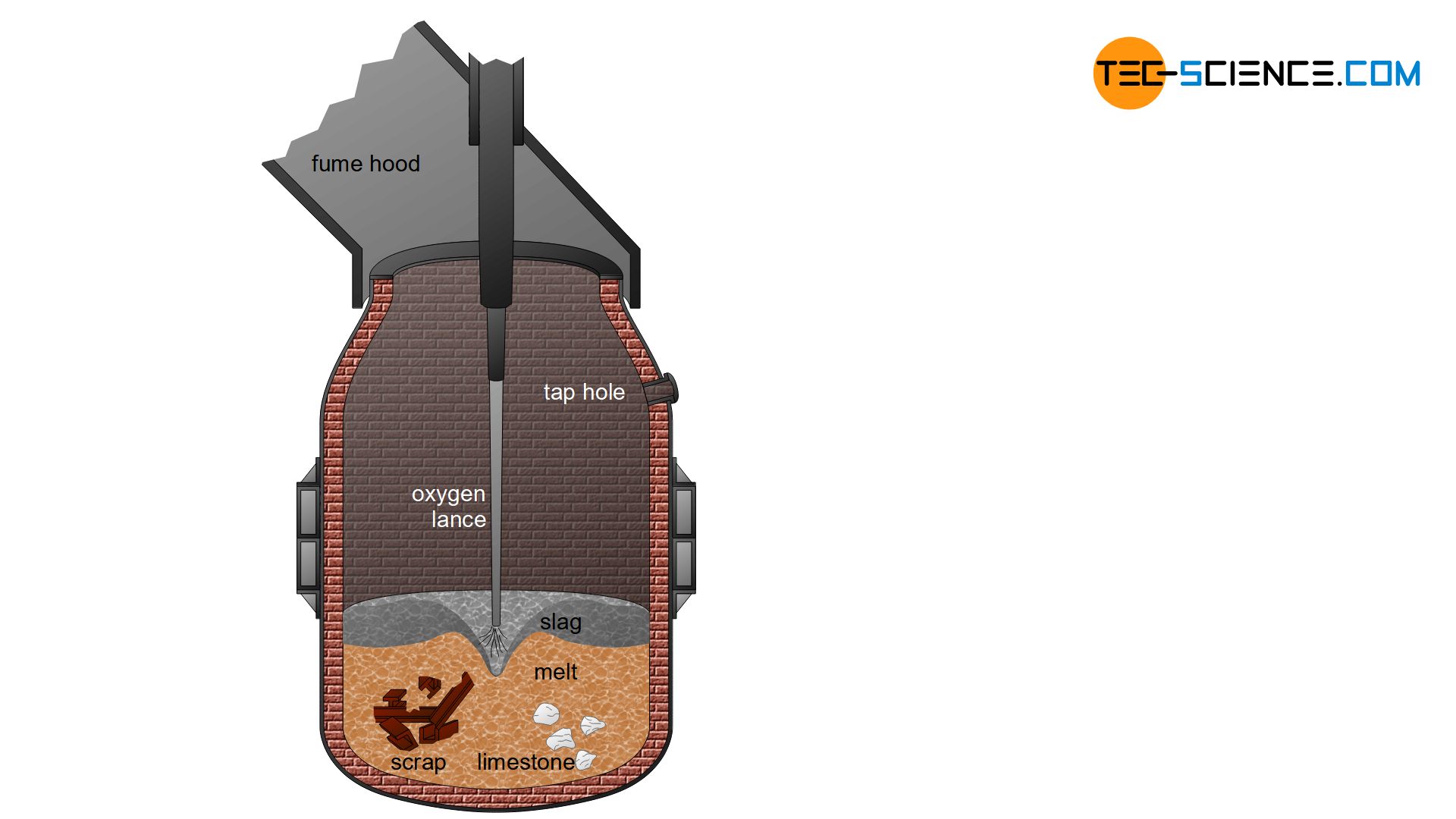
In the oxygen converter procedure, the pig iron is first filled into huge crucibles with a capacity of approx. 300 t, and so-called converters. Oxygen is then blown through a h2o-cooled copper lance onto the pig fe cook. This process was kickoff developed in the Austrian cities of Linz and Donawitz, which is why it is likewise known as the Linz–Donawitz-steelmaking (LD-steelmaking).
The carbon and undesirable elements are oxidized out of the pig iron by the injected oxygen. The melt is mixed by the loftier bravado force per unit area and the fierce oxidation that sets the pig fe cook in motility. With the aid of lime fluxes, the oxides formed are then bound in a basic slag or converted to the gaseous country.
The oxidation of the accompanying elements takes place via the formation of liquid iron oxide (\(FeO\)), which is formed by oxygen (\(O_2\)) on the sus scrofa iron (\(Fe\)):
\begin{marshal}
\label{eisenoxidation}
&Fe ~+~ O_2 &&\rightleftharpoons~ FeO ~+~ O \\[5px]
\end{marshal}
The oxidized iron and then reacts with the accompanying elements, whereby these themselves are oxidized due to the greater oxygen analogousness, while the iron oxide is reduced again. The chemical equilibrium equations of the most important accompanying elements are:
\begin{align}
\characterization{konverterverfahren}
&FeO ~&&+~ C &&\rightleftharpoons~ Fe && +~ CO \\[5px]
five~&FeO &&~+~ 2~P &&\rightleftharpoons~ 5~Fe && +~ P_2O_5 \\[5px]
&FeO &&~+~ Mn &&\rightleftharpoons~ Fe && +~ MnO \\[5px]
2~&FeO &&~+~ S &&\rightleftharpoons~ 2~Fe && +~ SO_2 \\[5px]
\characterization{konverterverfahren_ende}
ii~&FeO &&~+~ Si &&\rightleftharpoons~ ii~Iron && +~ SiO_2 \\[5px]
\stop{align}
The extremely exothermic oxidation of the accompanying elements ultimately resembles a combustion process. The temperature of the squealer atomic number 26 cook rises from 1250 °C to over 1600 °C. For this reason, approx. 20 % iron scrap is added for cooling in lodge to protect the converter lined with stones from excessive temperatures. Atomic number 26 ore or sponge iron (from the direct reduced fe process) can too exist used for cooling.
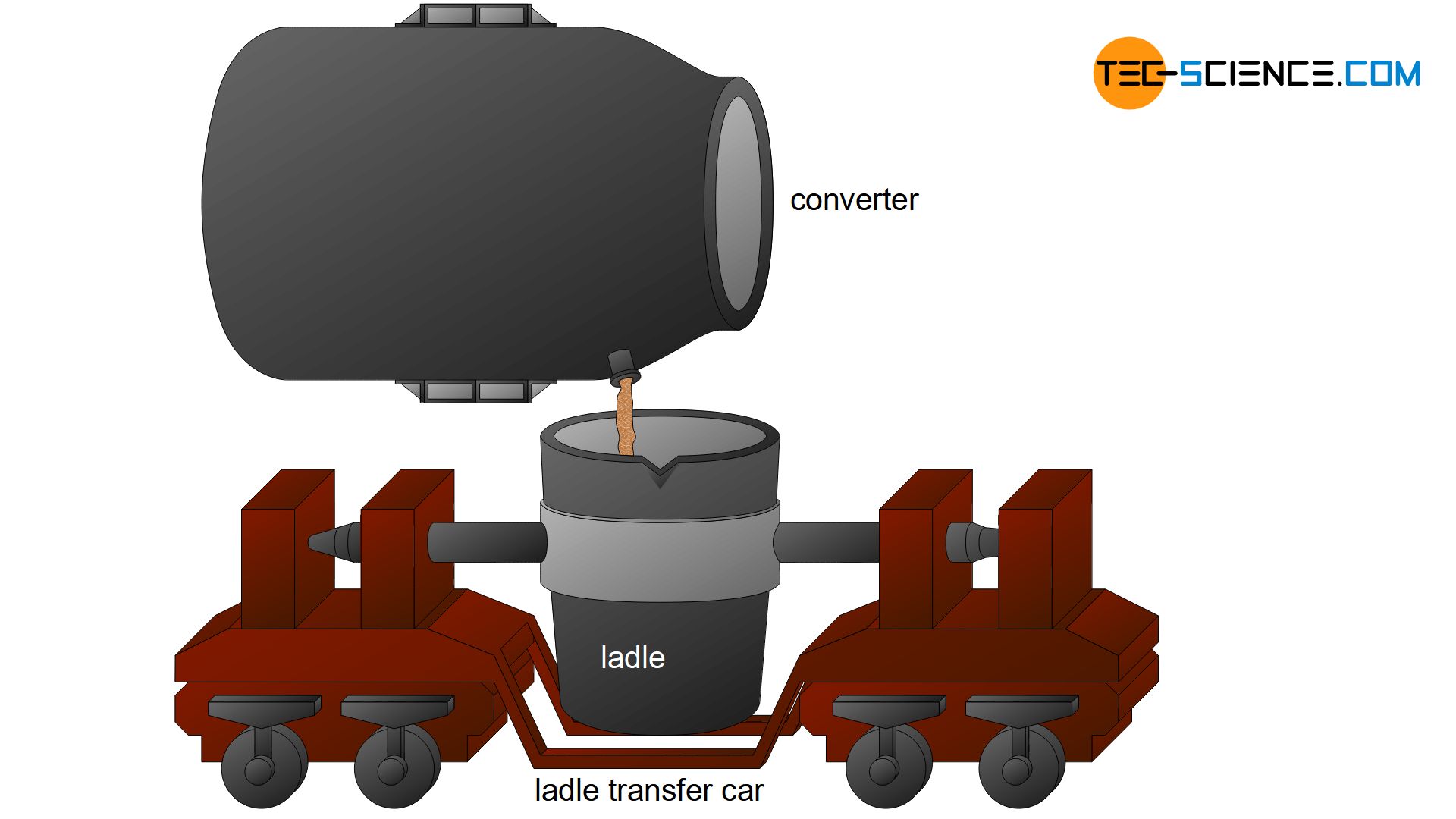
Afterwards xx minutes of oxygen supply, the decarburized cook is then called rough steel. For farther refinement, the rough steel is poured into ladles. This further handling is also referred to as secondary metallurgy and is dealt with in the following chapter.
With bones oxygen steelmaking (oxygen converter process), oxygen is blown onto the liquid cook and carbon and other elements are oxidized! Scrap is added for cooling!
Source: https://www.tec-science.com/material-science/steel-making/from-pig-iron-to-crude-steel/

0 Response to "How To Turn Iron Into Steel"
Post a Comment